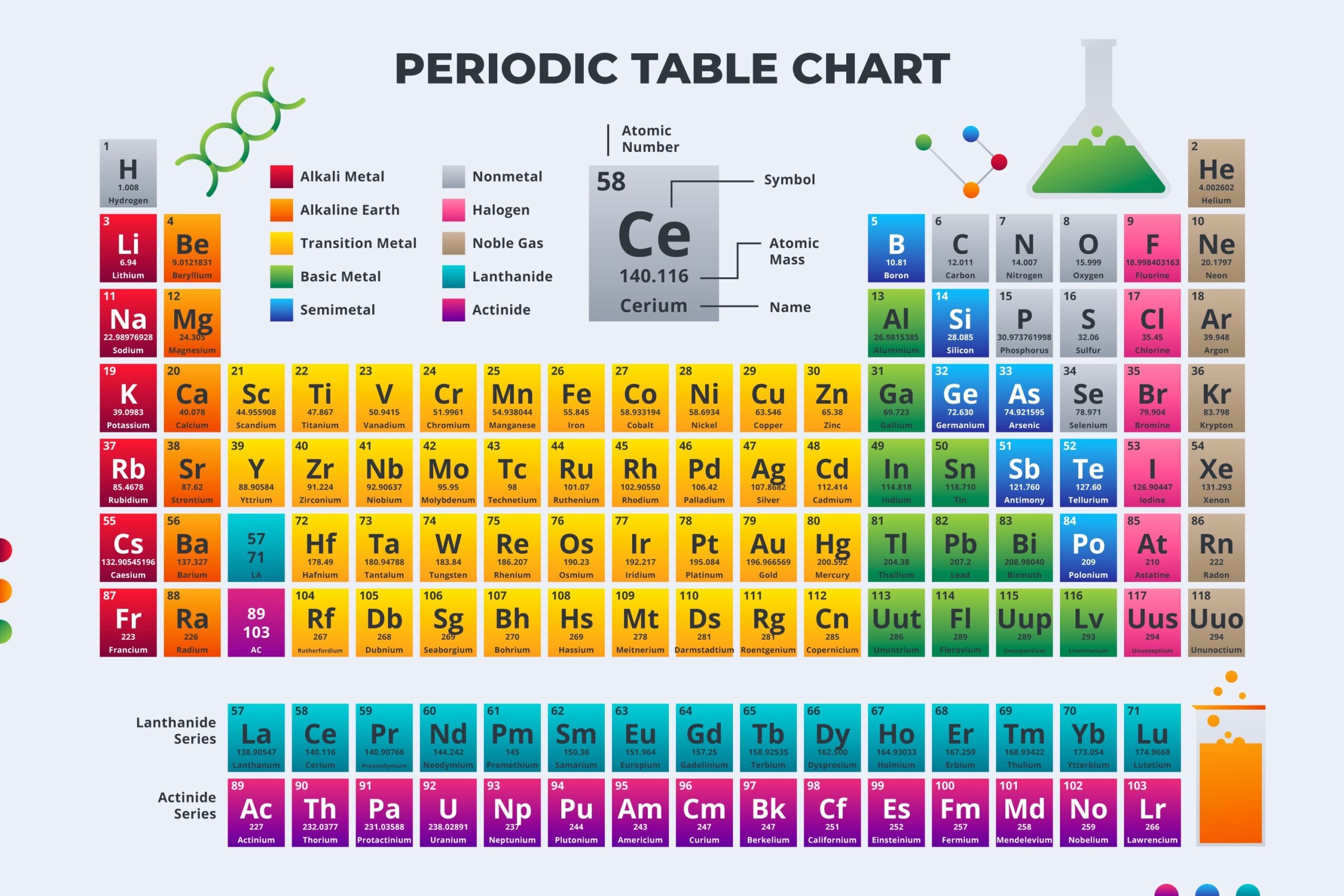
The Periodic Table of Elements: Introduction
The periodic table of elements stands as one of chemistry’s greatest achievements and serves as the foundation for understanding all matter around us. This amazing chart organizes all known elements in the universe, revealing patterns that help scientists predict how different substances will behave. Whether you’re a student just starting chemistry or someone curious about the building blocks of our world, this guide will take you through everything you need to know about the periodic table and its fascinating elements.

The Amazing Story Behind the Periodic Table of Elements
The periodic table has a rich history that spans over 150 years of scientific discovery. Before 1869, scientists knew about 63 different elements, but they had no clear way to organize them. Several brilliant minds attempted to create order from this chemical chaos.
The breakthrough came from Dmitri Mendeleev, a Russian chemist who changed science forever. On March 1, 1869, Mendeleev completed his revolutionary work that would become the modern periodic table. What made his approach special was that he arranged elements not just by atomic weight, but also by their chemical properties.
Mendeleev’s genius lay in his bold predictions. When his table showed gaps, he didn’t ignore them – instead, he predicted that unknown elements would fill these spaces. His confidence proved remarkable when three elements he predicted – scandium, gallium, and germanium – were later discovered with properties exactly matching his predictions.
Before Mendeleev, other scientists made important contributions. Antoine Lavoisier began classifying elements by properties in 1789. Johann Döbereiner discovered “triads” of elements with similar properties in 1817. John Newlands noticed that every eighth element had similar properties, calling this the “law of octaves”. However, Mendeleev’s table succeeded where others failed because it could predict new elements and correct atomic weights.
The discovery wasn’t made in isolation. The 1860 Karlsruhe Conference provided accurate atomic weights, giving Mendeleev the foundation he needed. This international collaboration shows how scientific progress builds on shared knowledge.

Understanding Groups and Periods: The Table’s Framework
The periodic table’s organization follows a logical pattern that reveals the secrets of atomic structure. Understanding groups and periods is essential for grasping how elements relate to each other.
Periods are the horizontal rows running across the table. There are seven periods, each representing elements with the same number of electron shells. For example, all elements in period 2 have two electron shells, while period 3 elements have three shells. As you move from left to right across a period, each element has one more proton than the previous one.
Groups are the vertical columns, and there are 18 groups in total. Elements in the same group share the same number of valence electrons – those crucial outer electrons that determine chemical behavior. This shared electron structure explains why elements in the same group have similar properties.
The table divides into distinct blocks based on which type of orbital holds the outermost electrons:
S-block (Groups 1-2): Contains alkali and alkaline earth metals
P-block (Groups 13-18): Includes metals, nonmetals, and noble gases
D-block (Groups 3-12): Houses the transition metals
F-block: Contains lanthanides and actinides
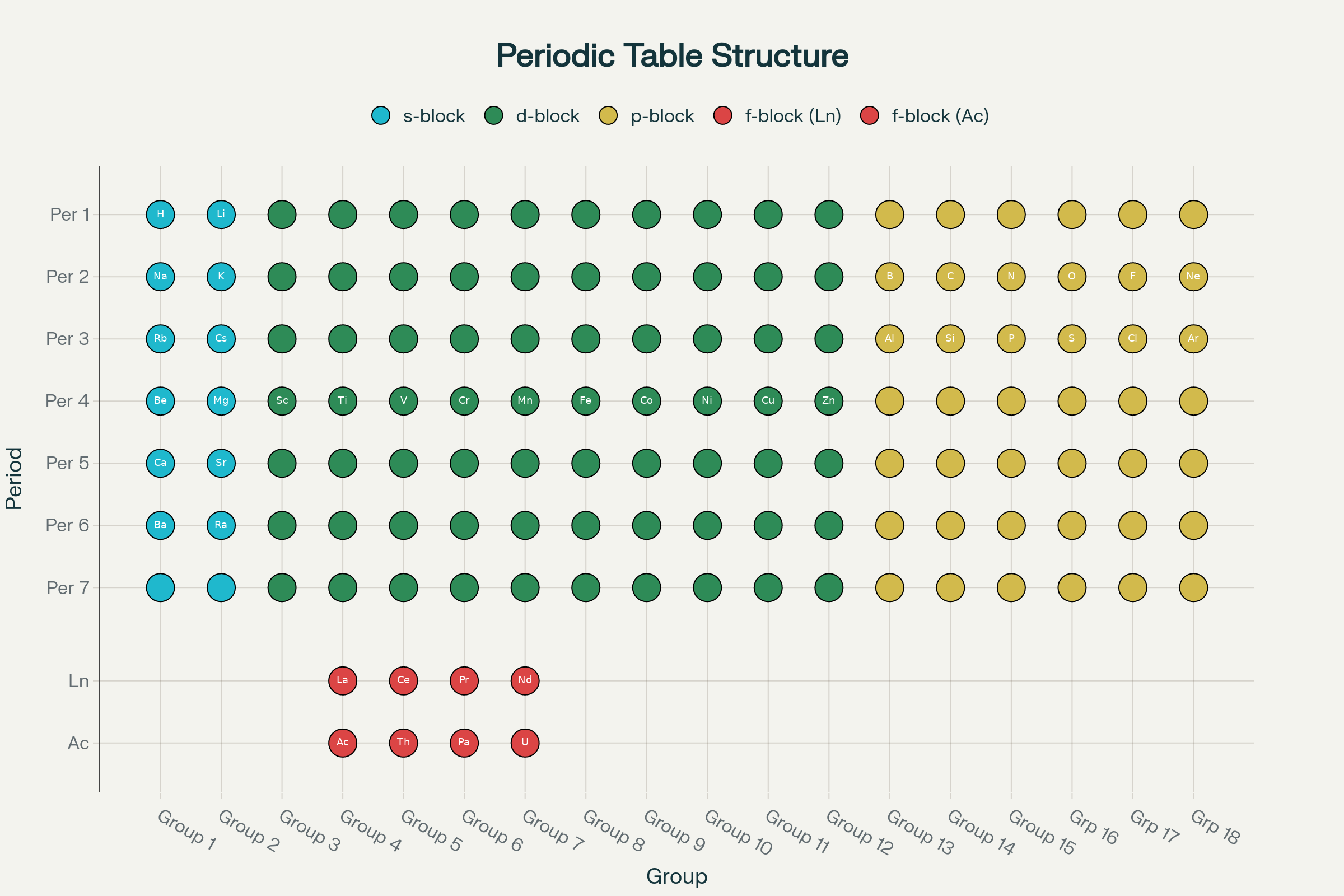
This organization isn’t random – it reflects the fundamental structure of atoms. As electrons fill different energy levels and orbital types, elements display predictable patterns in properties like atomic size, ionization energy, and reactivity. The beauty lies in how this arrangement lets us understand not just what elements are, but why they behave the way they do.
The Three Main Types of Elements: Metals, Nonmetals, and Metalloids
Elements fall into three major categories, each with distinct characteristics that determine their roles in our world.
Metals make up about 78% of all known elements and dominate the left side of the periodic table. These shiny, solid substances (except mercury, which is liquid) conduct heat and electricity excellently. Metals are malleable, meaning you can hammer them into thin sheets, and ductile, allowing them to be drawn into wires. They readily lose electrons, making them electropositive.
Common metals include iron for construction, copper for electrical wiring, and gold for jewelry. Aluminum’s lightness makes it perfect for aircraft, while titanium’s strength serves in medical implants. The alkali metals like sodium and potassium are so reactive they must be stored under oil to prevent explosive reactions with air and water.
Nonmetals occupy the upper right corner of the table and behave opposite to metals. They’re poor conductors of heat and electricity, often brittle, and tend to gain electrons during reactions. Nonmetals exist in various states: gases like oxygen and nitrogen, liquids like bromine, and solids like carbon and sulfur.
These elements are essential for life. Oxygen makes up nearly half of Earth’s crust and two-thirds of the human body. Carbon forms the backbone of all organic compounds. Halogens like chlorine serve as disinfectants, while nitrogen is crucial for plant fertilizers.
Metalloids bridge the gap between metals and nonmetals, showing properties of both. Silicon, the most famous metalloid, revolutionized technology as the foundation of computer chips. These “semi-metals” can conduct electricity under certain conditions, making them invaluable in the semiconductor industry.
Understanding how electrons arrange themselves around atomic nuclei explains why the periodic table works so perfectly. Electron configuration describes where electrons live in an atom and determines every element’s chemical personality.
Electrons don’t orbit randomly – they occupy specific energy levels called shells. The first shell can hold 2 electrons, the second holds 8, the third holds 8 (for elements 1-20), and so on. Electrons always fill the lowest available energy level first, following the Aufbau principle.
Within each shell, electrons occupy different types of orbitals:
s-orbitals: Spherical shapes holding up to 2 electrons
p-orbitals: Dumbbell shapes holding up to 6 electrons
d-orbitals: Complex shapes holding up to 10 electrons
f-orbitals: Even more complex, holding up to 14 electrons
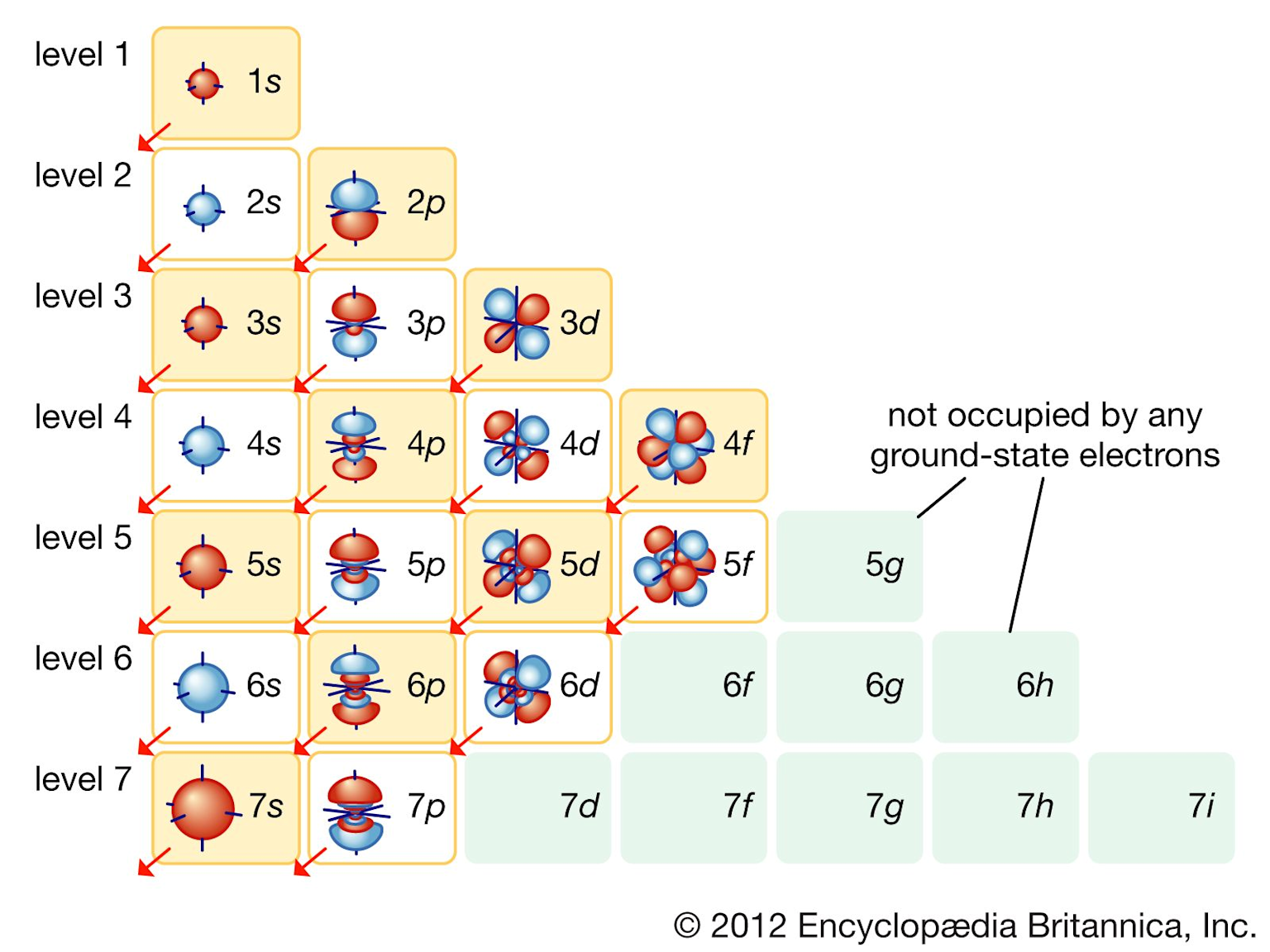 Electron shell and orbital filling diagram showing energy levels, orbitals, and their filling order with visual orbital shapes
Electron shell and orbital filling diagram showing energy levels, orbitals, and their filling order with visual orbital shapes This electron arrangement creates the periodic table’s patterns. Elements in the same group have identical outer electron configurations, explaining their similar behavior. For instance, all alkali metals have one electron in their outermost s-orbital, making them equally reactive.
As you move across a period, electrons gradually fill the same shell, but nuclear charge increases. This creates predictable trends: atomic radius decreases, ionization energy increases, and electronegativity rises. These patterns let chemists predict how elements will react, bond, and behave in compounds.
The noble gases showcase electron configuration’s power. Their complete outer shells make them incredibly stable and unreactive. This stability explains why helium balloons float safely and argon protects welding operations.
Fascinating Element Groups and Their Amazing Properties
Each group in the periodic table tells a unique story of elements working together based on their shared electron structure. Let’s explore the most important families and their remarkable characteristics.
Group 1: Alkali Metals are chemistry’s most reactive metals. Lithium, sodium, potassium, rubidium, cesium, and francium all have one valence electron, making them desperate to lose it and achieve stability. When dropped in water, they react violently, producing hydrogen gas and alkaline solutions – hence the name “alkali” metals.
These metals get more reactive as you go down the group. Lithium fizzes gently in water, sodium reacts more vigorously, while potassium burns with a beautiful lilac flame. Cesium is so reactive it can explode on contact with ice. Francium, the rarest naturally occurring element, would create an incredible explosion in water – if you could find enough of it.
Group 2: Alkaline Earth Metals include beryllium, magnesium, and calcium. With two valence electrons, they’re less reactive than alkali metals but still quite active. Magnesium burns with a brilliant white flame, making it perfect for fireworks and flares. Calcium builds strong bones and teeth, while also serving in construction as limestone and marble.
Groups 3-12: Transition Metals form the periodic table’s largest section. Iron builds skyscrapers and carries oxygen in our blood through hemoglobin. Copper’s excellent conductivity powers our electrical world. Gold’s chemical stability and beauty have made it precious throughout history. These metals can form colorful compounds and often have multiple oxidation states.
Group 17: Halogens are electron-hungry nonmetals with seven valence electrons. Fluorine, chlorine, bromine, and iodine desperately want one more electron to complete their outer shell. Chlorine disinfects swimming pools, fluorine strengthens teeth in toothpaste, and iodine prevents thyroid problems. Their reactivity makes them useful but dangerous – fluorine is so reactive it can eat through glass.
Group 18: Noble Gases represent atomic perfection with complete electron shells. Helium floats balloons and makes voices squeaky. Neon creates colorful signs, argon protects metal during welding, and xenon powers spacecraft. Their chemical inertness made them “noble” – they rarely associate with other elements.
Frequently Asked Questions
Q: How many elements are in the periodic table?
A: Currently, there are 118 confirmed elements in the periodic table. Of these, 98 occur naturally on Earth, while the remaining 20 are artificially created in laboratories. The newest additions were elements 113, 115, 117, and 118, officially named in 2016.
Q: Why is it called the “periodic” table?
A: It’s called “periodic” because the properties of elements repeat in regular patterns or periods. The horizontal rows are called periods, and as you move across them, similar properties appear at regular intervals.
Q: What makes an element radioactive?
A: Elements become radioactive when their atomic nuclei are unstable due to having too many protons or neutrons. These unstable nuclei spontaneously decay, emitting radiation to become more stable. All elements beyond uranium (element 92) are radioactive.
Q: Which element is most abundant in the universe?
A: Hydrogen is the most abundant element in the universe, making up about 75% of all ordinary matter. It’s the simplest element with just one proton and one electron.
Q: What’s the rarest naturally occurring element?
A: Astatine is the rarest naturally occurring element on Earth. Scientists estimate there’s less than one ounce of it in the entire Earth’s crust at any given time.
Q: Why did Mendeleev leave gaps in his periodic table
A: Mendeleev left gaps because he predicted that undiscovered elements would fill those spaces. His confidence was vindicated when gallium, scandium, and germanium were discovered with properties exactly matching his predictions.
Q: Which elements are essential for human life?
A: About 25 elements are essential for human life. The most important are hydrogen, carbon, nitrogen, and oxygen, which make up 99% of the human body’s mass. Others include calcium for bones, iron for blood, and iodine for the thyroid gland.
Q: What makes noble gases so special?
A: Noble gases have complete outer electron shells, making them extremely stable and unreactive under normal conditions. This chemical inertness makes them perfect for applications requiring non-reactive environments.
Conclusion
The periodic table represents one of humanity’s greatest intellectual achievements, transforming our understanding of the natural world. From Mendeleev’s brilliant insights in 1869 to today’s 118 confirmed elements, this elegant chart continues to guide scientific discovery and technological advancement.
We’ve explored how the table’s structure – with its groups, periods, and blocks – reflects the fundamental architecture of atoms themselves. The three main categories of elements each serve unique roles: metals provide structure and conductivity, nonmetals enable life processes, and metalloids power our digital age.
Understanding electron configuration reveals why elements behave as they do, while exploring different element groups shows the incredible diversity of matter in our universe. From the explosive reactivity of alkali metals to the serene stability of noble gases, each element family tells its own fascinating story.
The periodic table isn’t just a historical artifact – it’s a living document that continues to grow as scientists discover new elements and applications. These fundamental building blocks of matter shape everything from the stars above to the cells in our bodies, making the periodic table truly universal in its significance.
As we look to the future, researchers continue pushing the boundaries, attempting to synthesize elements 119 and 120. Who knows what new discoveries await? The periodic table reminds us that science is an ongoing adventure, where each new element adds another piece to our understanding of the cosmos.